Clinuvel Pharmaceuticals has shared some encouraging early data from its latest stroke study, with results suggesting its experimental treatment, PRÉNUMBRA Instant, could offer hope to patients who aren’t eligible for traditional stroke therapies.
The study, known as CUV803, involved nine patients with varying levels of stroke severity — from mild to moderate-to-severe — all of whom were treated at European specialist centres. Importantly, these patients couldn’t receive the usual clot-busting treatments like thrombolysis or thrombectomy, which meant options were limited.
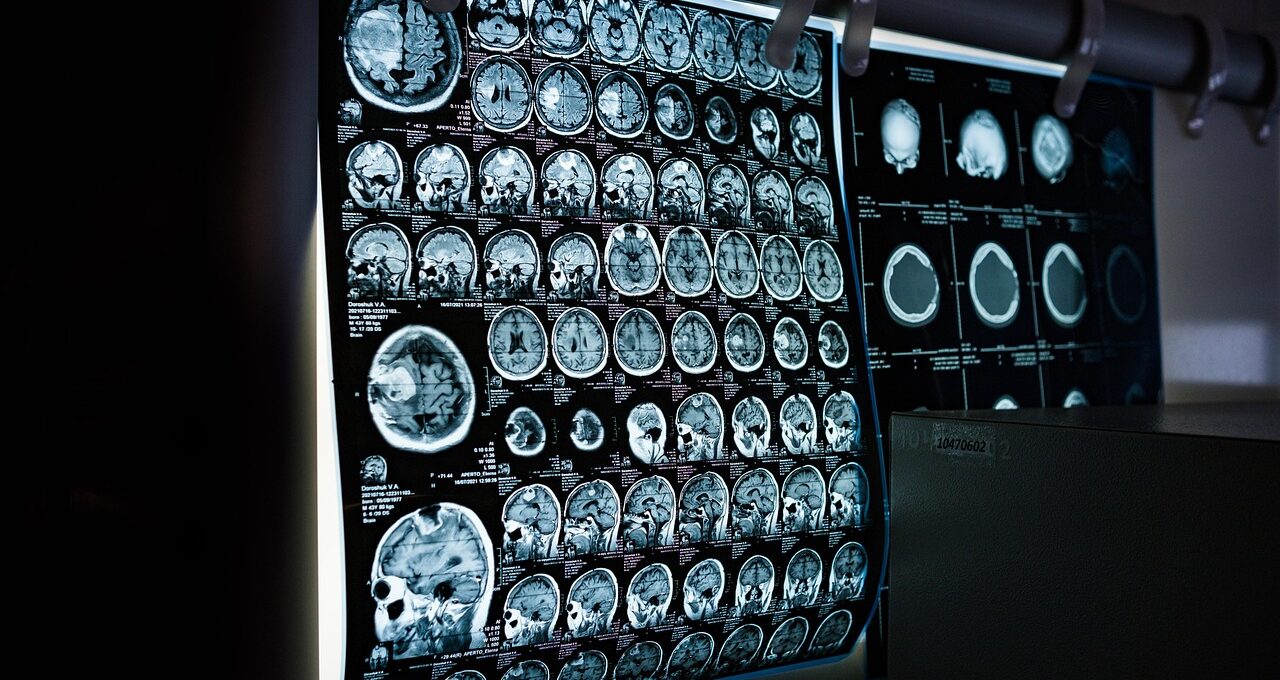
Over a treatment course of up to five days, patients received afamelanotide, the active compound in PRÉNUMBRA, which has shown neuroprotective potential. The key takeaway? The treatment was well tolerated across the board, with only mild and short-lived side effects. By day 42, eight out of nine patients showed improvement in their symptoms and neurological function, and imaging revealed either stability or a reduction in stroke-related brain damage in two-thirds of them.
While two patients with severe strokes sadly passed away from unrelated complications, the broader safety profile was consistent with what’s been seen in previous uses of afamelanotide.
Looking at Clinuvel’s stroke program overall — which now includes 15 patients across two studies — the data paints a positive picture: 87% of patients showed functional improvement, and nearly 67% experienced stable or reduced brain damage on scans.
Despite the promising signals, Clinuvel has confirmed it’s pressing pause on the stroke program for now, shifting its focus toward vitiligo, porphyrias, ACTH development, and cosmetic applications. Still, the company believes this data could help guide future research — and potentially give stroke patients new treatment options down the track.
CLINUVEL’s Chair, Prof Jeffrey Rosenfeld said:
“With the CUV801 and CUV803 studies we have generated a small, but encouraging, data set that demonstrates the drug’s safety profile in patients with strokes of varying degrees of severity, as well as first indications that patients may benefit from treatment. While the stroke program is not an immediate focus for the business, I expect our teams will be able to use these data to support future decision making that may result in positive outcomes for patients,”